
Ground equipment to eliminate build-up of static charge. Since the outer shell is not filled and the ionization potential and the electron affinity are 12.61 and 1.46 eV, respectively, the oxygen atom usually acquires electrons in the course of formation of chemical compounds and has a negative effective charge.
#Diatomic oxygen charge free
After this, the hydrogen atom no longer has any free electrons, and accordingly, the positive charge.
#Diatomic oxygen charge series
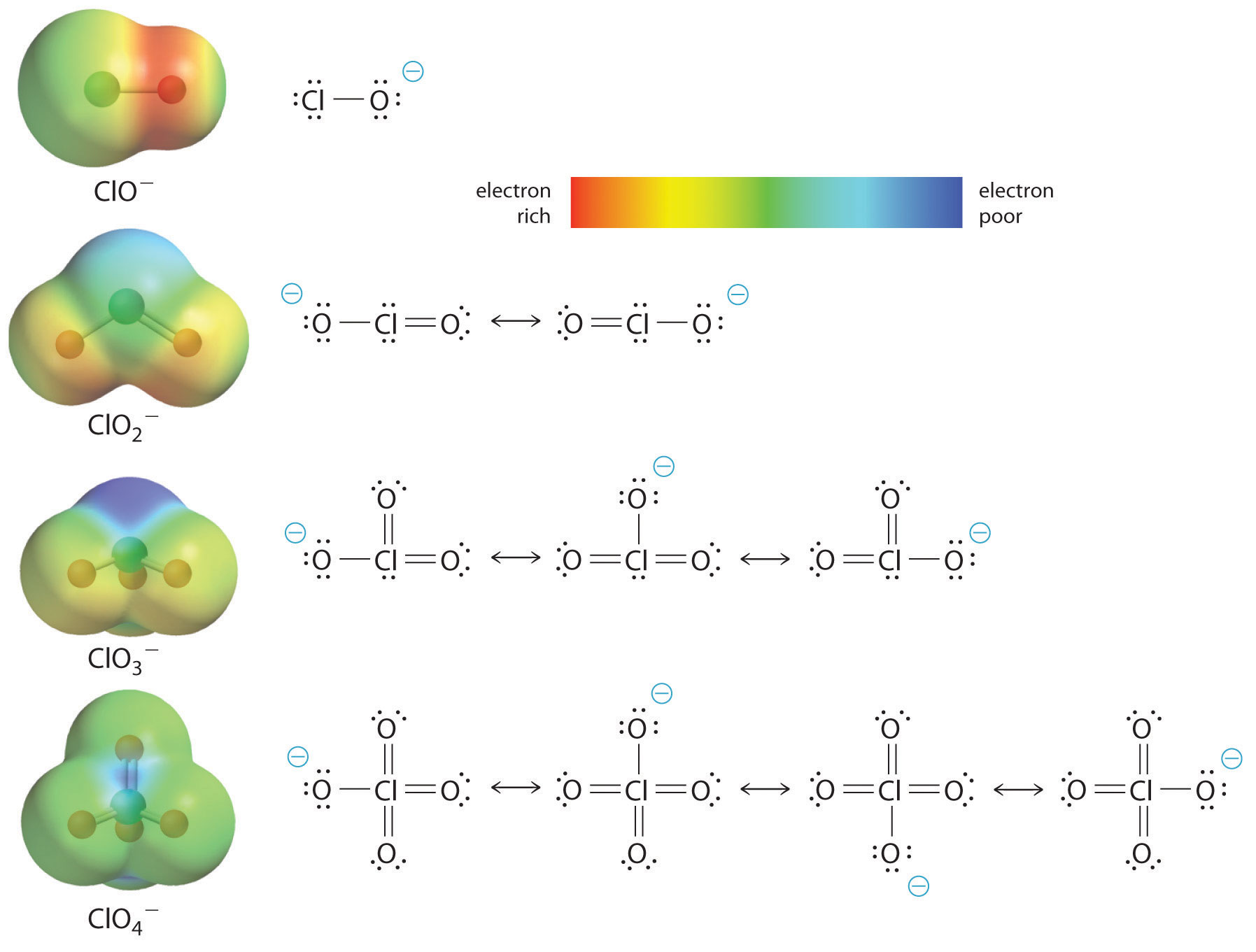
form a series of polyatomic ions with oxygen (all having the same charge).
In the lewis structure of O2 molecule, a double bond is located between oxygen atoms and. Dioxygen is a diatomic oxygen, a gas molecular entity and an elemental molecule. At the same time, hydrogen gives oxygen its only electron. Ionic compounds are compounds composed of ions, charged particles that form. \), and \(Cu^+\).įor example, you would expect the electron configuration of Cu to be: 1s 22s 22p 63s 23p 64s 23d 9 (paramagnetic, 1 unpaired electron) and when it loses one electron to form the Cu + with the following electron configuration: 1s 22s 22p 63s 23p 64s 23d 8(paramagnetic 2 unpaired electrons). Oxygen is a diatomic molecule and contains only two oxygen atoms.


 0 kommentar(er)
0 kommentar(er)
